Background. Antiphospholipid syndrome (APS) is an autoimmune disease with thrombotic and obstetric complications arising via a model of immunothrombosis. Patients may present with a spectrum of phenotypes, including thrombotic (tAPS), obstetric (oAPS), or catastrophic/microvascular APS (C/MAPS), while others may have antiphospholipid antibodies (aPL) without disease manifestations. The mechanisms underlying the development of these diverse phenotypes remain uncertain. Proteomic profiling was used in other thrombotic and microvascular disorders to highlight potential mechanisms of disease pathogenesis and may have a role in understanding the pathophysiology of APS. We performed multiplex plasma proteomic profiling in aPL-positive patients with different clinical phenotypes to gain a greater understanding of potential immunothrombotic mechanisms in the pathogenesis of APS.
Methods. We utilized samples from APS ACTION Registry. The inclusion criteria were positive aPL per Updated Sapporo Classification Criteria tested within one year prior to the enrollment. Multiplex proteomic profiling measuring approximately 7,000 unique proteins (SomaLogic; Boulder, CO, USA) was performed on 40 primary aPL-positive patient plasma samples (10 each of tAPS with/without oAPS, oAPS only, C/MAPS, and positive aPL without APS classification), and 10 of healthy controls. Differentially abundant proteins among all phenotypic groups and in pairwise comparisons were determined by applying ANOVA and t-tests to log-normalized data, respectively, with p-values <0.05 considered statistically significant (Qlucore Omics Explorer, Lund, Sweden). Tests were adjusted for false discovery (q<0.1) to minimize the likelihood of false positives. Pathway enrichment analysis was performed using Metascape (https://metascape.org) and Ingenuity IPA (Qiagen, Venlo, Netherlands) platforms.
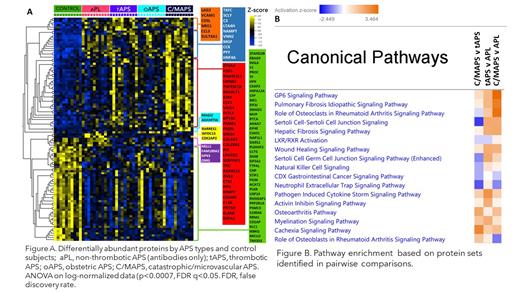
Results. The median age of patients was 48 years; 30% were men, 70% had triple aPL-positivity, and no one had a concurrent diagnosis of lupus. A set of concordant and differentially abundant proteins clustered patients with 4 APS clinical phenotypes and controls (Figure A) with a high statistical significance (p<0.0007) and a high false discovery confidence (q<0.05). Proteins in the identified set belonged to several highly enriched pathways such as neutrophil degranulation (p<10 -10), humoral immune response (p<10 -9), coagulation (p<10 -6), and alternative complement (p<10 -5) [data not shown].
Pathway enrichment analysis of protein sets identified in pairwise comparisons of APS phenotypes (aPL vs C/MAPS, aPL vs tAPS, tAPS vs C/MAPS) revealed involvement of several common pathways associated with inflammation, collagen and fibroblast signaling, cellular and cytoskeletal activation, humoral immune response, myeloid and effector cell differentiation and recruitment, and immunothrombosis such as Pathogen-Induced Cytokine Storm, Neutrophil Extracellular Trap (NET), and collagen-induced platelet-activating GP6 signaling (Figure B).
For several pathways, the measure of activation related to increased or predicted to be increased proteins annotated within a particular pathway positively correlated with the clinical “distance” between APS subtypes, from the most clinically related C/MAPS vs tAPS to the most distant aPL vs C/MAPS suggesting an “evolution” from one phenotype to a more severe in terms of activation of specific pathways. Specifically notable is the higher activation of the NET signaling pathway in the aPL than in C/MAPS phenotype suggesting that the presence of aPL antibodies activates this immunothrombotic pathway.
Conclusions. Plasma proteome of APS subtypes is characterized by alteration in several cellular processes, particularly receptor signaling, signal transduction, regulation of cellular differentiation, neutrophil, complement, coagulation and cytokine activation notable in all individuals with aPL-positivity. Pathways activated in the non-thrombotic (aPL) phenotype and escalating activation of several pathways from non-thrombotic to the most thrombotic (C/MAPS) phenotype provide important data for the understanding of APS pathogenesis and informing the model of APS-related immunothrombosis.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal